Beadbeaters: Indispensable Tools for the Latest Cell Homogenization Applications
Enabling solutions for modern molecular and genetic techniques when accuracy, precision, and quality are essential
As a technology, beadmilling—commonly called beadbeating—has grown to enable a multitude of workflows in the lab. The technique allows users to tackle applications in which sensitivity, precision, and speed of homogenization are paramount. The ability to adjust the bead type and size, shaking pattern, and the scale has made the technique extremely versatile as well. These abilities offer advantages for modern molecular techniques such as PCR (Polymerase Chain Reaction), NGS (Next-Generation Sequencing), and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) in which the efficiency of cell homogenization directly affects downstream success of the techniques.
Background
Beadmilling or beadbeating originally emerged as an approach to address drawbacks of mechanical disruption techniques while enabling access to a growing variety of cells and tissues. Since its inception, beadbeating has evolved to become the tool of choice for rapid, efficient disruption of cells and tissue samples in the lab. Importantly, the technique offers advantages for producing high yield homogenates for sensitive applications, such as those used in molecular and genetic investigations involving proteins, DNA, and RNA.
Beadbeating basics
How does beadbeating work? Wet beadbeating entails mixing an equal volume of minute glass, ceramic, or steel beads with a buffered suspension of microorganisms, cell, or tissue pieces. The mixture is placed in a screw-cap microvial and mechanically agitated, stirred, or vortexed at high speed. Cell disruption occurs by the crushing action of the colliding beads. Compared to ultrasonication or high-pressure methods, beadbeating is relatively low in shearing forces. Sample heating can be easily controlled by incubation on ice.
Optimal bead size and composition is adjusted according to the cell sample size and type. For instance, the bead diameter of choice for bacterial cells is 0.1 mm beads where the ideal bead diameter for yeast is 0.5 mm. Glass beads are preferred for microbial cells, whereas higher density ceramic or steel beads are best for tougher cells and tissue. When using steel beads, common microvials can be swapped to more durable stainless steel or reinforced polypropylene microvials to avoid cracking under high impact agitation.
The high agitation speed and the shaking pattern of shaking type bead mills influence on the rate and efficiency of cell lysis. Relative to the vial orientation, the shaking pattern is commonly in parallel or in a figure-8 motion.
By selecting the appropriate bead size and density, as well as moderating the rate of agitation, a variety of cell types can be lysed. The beads quickly settle out by gravity and the homogenate is removed by pipette. Centrifugation recovers the intact cells and debris, producing a soluble, cell-free extract as a supernatant. Under milder beadbeating conditions, functional organelles such as mitochondria and nuclei can be recovered and isolated using gradient centrifugation. At high shaking speeds, proteins and nucleic acids can be recovered at high yields, with minimal denaturation and shearing.
Cell lysis takes place in sterile, single-use vials. Thus, the chance for cross contamination between samples is eliminated. This allows PCR and other sensitive techniques to be performed without threat of contamination. These advantages are especially important for modern quantitative polymerase chain reaction (qPCR), next-generation sequencing (NGS), and gene-editing (CRISPR) applications.
Beadbeating for molecular and genetic applications
PCR and quantitative qPCR
In these common techniques, cell homogenization allows the release of genomic DNA or RNA from cells, making it accessible for subsequent amplification and analysis. Whether enlisting standard PCR or quantitative PCR (qPCR), cell homogenization is a critical step in the process. Important Considerations include:
Yield: Complete disruption of cell walls and membranes is essential to release this genetic material needed for PCR analysis. The thoroughness of lysis directly affects the yield of genetic material, which is especially important where sample volumes are limited or target nucleic acids concentrations are low. Beadbeating duration and bead type offer adjustable variables that can be optimized to produce the highest yields possible.
Sensitivity and Reproducibility: A high level of uniformity or precision in homogenization helps minimize contamination from cellular debris that could interfere with PCR amplification. Beadbeating precision helps ensure there are accurate amounts of genetic material in each sample. This is especially important in quantitative techniques such as qPCR. Beadbeating can easily be multiplexed to process multiple samples in parallel, thus further maximizing reproducibility.
Quality: Optimization of PCR can be influenced by the quality (and quantity) of the template DNA or RNA. The ability to lyse cells with minimal shearing, and heat generation ensures the quality of DNA harvested during the beadbeating process.
Time and Cost Efficiency: Many downstream applications such as sequencing, genotyping, or gene editing rely on accurate and reproducible DNA and RNA extraction methods. Efficient cell homogenization streamlines the overall PCR workflow and reduces the time of cost of sample processing. Furthermore, high efficiency workflows minimize the need for sample repetition or troubleshooting, thereby saving time and resources. The simplicity and capabilities of high throughout beadbeating reduces processing time and provide significant cost savings.
Next Generation Sequencing
DNA and RNA preparation is an essential process in NGS workflows as well. Many of the same requirements described above also apply, including maximizing yield and uniformity while minimizing contaminants. Additional considerations include:
Sequence Coverage and Depth: The better the yield and quality of genetic material following lysis, the more successful the sequencing coverage. This is particularly important for detecting, with high fidelity, rare genetic variants or low-abundance transcripts.
Genetic Library Complexity: Many NGS applications rely on the use of complex genetic libraries. Sufficient complexity increases the chances of capturing diverse genetic information during sequencing. Yield and quality of genetic material following cell lysis directly impact these odds.
Efficiency and Compatibility: Effective cell lysis ensures genetic material is compatible with the chosen sequencing platform. Efficient beadbeating streamlines the overall NGS workflow thereby saving time and resources.
CRISPR
During gene-editing processes such as CRISPR, components are transferred into cells where they can perform site-specific DNA cleavage and repair. Special considerations include:
Editing Analysis: Efficient homogenization is important for extracting DNA or RNA from the edited cells and analyzing the outcomes. The presence of indels (insertions or deletions) or specific genetic modifications can be determined using PCR, sequencing, or functional assays.
High-throughput Screening: Consistent cell homogenization is crucial in processing large numbers of cells in high-throughput gene editing and CRISPR screening experiments.
The ability to adjust time duration, bead size and material, and other parameters like agitation pattern assists in optimizing beadbeating yields according to the cell type and application. Minimizing loss due to heat, denaturation, and shearing can help maximize library complexity and produce genetic material that can be compatible with successful NGS and CRISPR workflows. Beadbeating offers the ability to perform sterile lysis in discrete microvials which helps ensure samples are free from contaminants and potential cross contamination between samples.
Beadbeater products and solutions
In 1979 BioSpec Products was the first to introduce lab-scale bead mill cell disrupters and it has dominated the market for about 40 years. The line of Mini-BeadBeater devices utilize agitation and flexible designs to accommodate a wide range of volumes and sample capacity.
The Mini-BeadBeater-16 is a high-energy cell disruptor designed to accommodate 4 to 16 samples at a time in screw-cap microvials. Cells are completely homogenized in two–three minutes in 0.1 to 1 ml of extraction medium. In the presence of nucleic acid extraction media such as phenol, Gu-SCN, or a commercial kit solution, DNA and RNA are recovered in the highest possible yield—ideal for PCR and other genetic techniques. Because beads and microvials are disposable, there are no cross-contamination concerns when homogenizing multiple samples.
The Mini-Beadbeater-24 (shown right) disrupts up to 24 microbial or tissue samples in 2 ml microvials with better than 95 percent efficiency. Cells are disrupted quickly and safely in the sealed system. The apparatus is easy to clean, has a small footprint and is essentially maintenance free. Compared to the Mini-Beadbeater-16, the Mini-24 adds advanced electronics for enhanced motor function, variable speeds of 2400–3800 rpm and a 30 percent increase in microvial capacity. This setup allows the device to be further tuned to suit a variety of applications including nucleic acid preparation for sensitive techniques.
More information
The Mini-BeadBeater-96 (shown right) is a high-energy, high-throughput cell disrupter. It can process one 2 ml deep well microplate (96 sample capacity), two 1 ml deep well microplates (192 sample capacity), or using the included 45 capacity polypropylene rack, 0.6, 1.5, or 2 ml microvials. The system has high-throughput capacity to process thousands of samples within the span of a day. This setup is ideal for nucleic acid library prep or high throughput sequencing applications.
 The Mini-BeadBeater-Plus
(shown right) is a high-energy, single-sample bead mill designed for small
labs. The Mini-BeadBeater-Plus features a programmable timer, a powerful
brushless motor, and interchangeable 115/230 voltage compatibility. This
option is well-suited for nucleic acid minipreps or small-scale analytical
applications.
The Mini-BeadBeater-Plus
(shown right) is a high-energy, single-sample bead mill designed for small
labs. The Mini-BeadBeater-Plus features a programmable timer, a powerful
brushless motor, and interchangeable 115/230 voltage compatibility. This
option is well-suited for nucleic acid minipreps or small-scale analytical
applications.
For much larger batches of microbial suspensions, BioSpec Products offers a preparative lab BeadBeater. This device differs in mechanics from shaking type beadbeaters in that beads are agitated with a rapidly turning rotor rather than by shaking.
 The BeadBeater laboratory-sized rotor-type beadbeater (shown right) can
disrupt 5 to 80 g (wet weight) of microbial cells suspended in 250 ml of
extraction media in three to five minutes. Smaller chamber attachments are
available to process 50- or 15-mL batches of cell suspension. The system uses a Teflon impeller rotating at 15,000 rpm to force
thousands of beads to violently collide in a special, clover-leaf-shaped
vessel. Cells are disrupted quickly, efficiently, and safely in this sealed
system. The BeadBeater is easy to clean, has a small footprint, requires no
auxiliary supplies or equipment and is essentially maintenance free.
The BeadBeater laboratory-sized rotor-type beadbeater (shown right) can
disrupt 5 to 80 g (wet weight) of microbial cells suspended in 250 ml of
extraction media in three to five minutes. Smaller chamber attachments are
available to process 50- or 15-mL batches of cell suspension. The system uses a Teflon impeller rotating at 15,000 rpm to force
thousands of beads to violently collide in a special, clover-leaf-shaped
vessel. Cells are disrupted quickly, efficiently, and safely in this sealed
system. The BeadBeater is easy to clean, has a small footprint, requires no
auxiliary supplies or equipment and is essentially maintenance free.
To combat heat generation during the Beadbeating process, an ice-water jacket included with the BeadBeater helps keep this temperature increase under control. Superior control is achieved using an accessory stainless steel or aluminum sample chamber.
This BeadBeater is well-suited for scale-up and large-quantity nucleic acid applications.
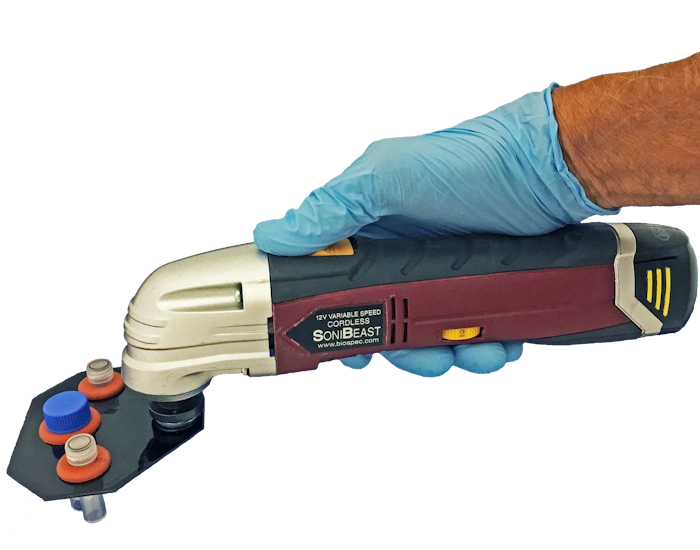 he handheld SoniBeast-JR (shown right) is a cordless, variable-speed
high-energy bead mill. While the SoniBeast-JR is targeted for
processing samples in the field or locations removed from the laboratory, it is
also an affordable choice for processing a limited number of samples
(<20/day) in the lab. Manually operated with a slide switch on the body of
the SoniBeast-JR's motor housing, the vial tray holds two 2 ml screw-top
polypropylene microvials or one 7 ml screw-top polypropylene vial. The
SoniBeast-JR comes with a smart recharging station powered by 115-volt
electrical input.
he handheld SoniBeast-JR (shown right) is a cordless, variable-speed
high-energy bead mill. While the SoniBeast-JR is targeted for
processing samples in the field or locations removed from the laboratory, it is
also an affordable choice for processing a limited number of samples
(<20/day) in the lab. Manually operated with a slide switch on the body of
the SoniBeast-JR's motor housing, the vial tray holds two 2 ml screw-top
polypropylene microvials or one 7 ml screw-top polypropylene vial. The
SoniBeast-JR comes with a smart recharging station powered by 115-volt
electrical input.
The setup is ideal for fast processing of single samples in remote applications.
Summary
Beadbeating has evolved to become a dominant technology for lab homogenization needs. The versatility and cost effectiveness make it ideal for any lab—from research to pilot-plant scale. Due to the technique’s effectiveness and versatility, beadbeating will undoubtedly see further widespread usage in PCR, next-generation sequencing, gene-editing techniques such as CRISPR, and other evolving molecular and genetic techniques.
Published in partnership with BioSpec Products.