Adding Depth to Your Research: A Look at 3D Cell Culture
By Suzanne Leech, PhD
For the last forty years, biologists have used traditional 2D cell culture to forge breakthroughs in cell biology. Although still very relevant, 2D culture can have its drawbacks. Cells grown on flat plastic surfaces behave differently to in vivo cells; they tend to spread themselves as flat as possible, have minimal cell-cell interactions and display different expression and morphogenesis. 2D cell cultures also have a much shorter life-span than 3D. Kristi Anseth, a bioengineer who leads a research group at the University of Colorado Boulder, put it to Science magazine simply: “3D culturing is more relevant to biology because the vast majority of cells in our bodies grow that way”.
3D culturing has become popular in the field of drug-screening and toxicity, as the cells respond to chemicals in a more ‘in vivo way’ than 2D cultures. But the technique also has applications in basic research. “The combination of biomaterials and state-of-the-art technologies for manipulating human 3D brain cultures has the potential to give rise to novel features in vitro and accelerate the study of human brain development and disease.” Sergiu Pasca, Department of Psychiatry and Behavioural Sciences, Stanford.
In recent years, huge steps have been made to standardize and manufacture equipment for 3D cell culture—making it cheaper, simpler and more reproducible.
The most popular commercially available materials for the two main types of 3D culture—Scaffold and Non-scaffold—are described below:
Non-scaffold 3D Cell Culture
Balls of interacting cells form ‘organoids’ or ‘organ spheroids’ that replicate tissues in vivo. There are three main techniques:
- The forced-floating method uses low adhesion polymer-coated culture vessels. It has been used to successfully grow human tumor spheroids. Creative Bioarray and Corning produce ‘Ultra-low attachment’ multi-well culture plates of various shapes and sizes. SBio produce the PrimeSurface® range offering dishes and multi-well plates of various size and well-shape. Thermofisher Scientific produces various sizes of ‘Nunclon Sphera’ plates and flasks. Lipidure®-coated vessels are available from AMS-bio in a range of dishes and plates. AMS-bio can provide the Lipidure® coating in powder form for the self-preparation of any vessel.
- The hanging drop method employs specially designed multi-well plates that, when inverted, turn the aliquot into a free-hanging drop. This method was found to be effective for culturing mammalian hepatocytes. Plates are available from Creative Bioarray and Sigma-Aldrich make the Perfecta3D® 384 well plate. Elplasia produce various sizes of multi-pore well that fit inside standard 6-well plates.
- Agitated suspension methods prevent cells adhering to the vessel surface by continuous movement. Rather than specialized vessels, the technique uses bioreactors e.g. the Synthecon RCCS bioreactor system. The Biotek 3D Perfusion Bioreactor system accommodates multi-well plates.
Scaffold-based Cell Culture
The use of scaffolding allows cell spheroids to adhere to a non-cellular matrix, as often happens in vivo. The spheroids can grow larger than in non-scaffold cultures and display in vivo structures, cell-cell interaction and differentiation. The pores or channels allow for the movement of nutrients, oxygen, and cell signalling molecules and vastly increase the surface area.
Scaffolds have been used for growing tissues for implantation and are now being used for drug-screening and research. There are many commercially available materials, just a few are outlined here:
- Hydrogel scaffolds are the most popular scaffold types as they mimic the extracellular matrix (ECM) and are biological in origin. Scientists can seed the liquid form with cells before solidification for even distribution. Corning produces Matrigel™ from mouse tumors that over-secrete extracellular matrix proteins. Matrigel™ has been used to observe the growth of prostate cancer cells. However, Matrigel™ composition may vary between batches and have an undefined biochemistry. AlgiMatrix 3D Culture System by ThermoFisher is an animal-free, chemically defined porous matrix that comes pre-formed in multi-well plates. Scientific Lifecore Biomedical make Coregel® from a common ECM polysaccharide.
- Biopolymer scaffolds can be impregnated with growth factors, hormones or drugs. SpongeCol® is a collagen-based columnar scaffold, and Go Matrix is a ridged gelatin-based scaffold, both from Sigma-Aldrich.
- Hard polymer scaffolds are made from artificial materials such as ceramic, glass or metal and are used for tissue engineering. Cells are seeded by diffusion and penetration can be poor, and imaging and analysis is difficult. The larger scaffolds are generally unsuitable for basic cell culture. However, Sigma-Aldrich produce nanofibre-scaffolds for dishes and plates, which are transparent—allowing for in situ staining and imaging.
Organ-On-A-Chip
Another application of 3D cell culture is the innovative ‘organ-on-a-chip’ model. The system involves a small polymer platform and the properties of ‘microfluidics’: allowing precise control of the nutrients and chemicals reaching the cells. The ‘lung-on-a-chip’ uses human vascular endothelial cells and alveolar epithelial cells to model the capillary barrier of the lung. Micronit Microfluidics manufacture organ-on-a-chip platforms and the accessory kits and pumps. Elveflow® produce complete ‘plug & play’ microfluidic organ-on-a-chip packs with all the necessary hardware to get started.
Summary
So whether to use 2D or 3D cell culture will depend on the experiment or assay you wish to conduct, the cells you are using and your budget and deadline. Your project may even benefit from employing a combination of both techniques. As the materials for 3D methods become more accessible, easier to use and less expensive (and more studies using the technique are published in the literature) it is likely that the 3D-approach will become more and more popular in both basic biological research and drug-assay trials.
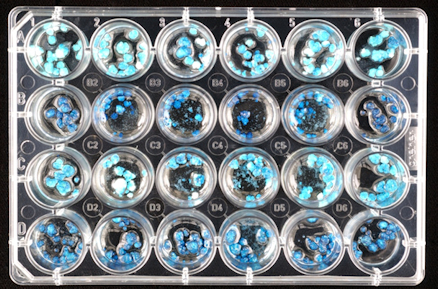
Top image: 24-well microtitre plate with tissue culture
https://www.scienceimage.csiro.au/tag/laboratories/i/3770/microtitre-plate/
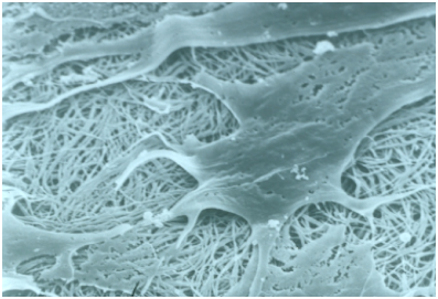
Lower image: Scanning electron image of cells interacting with collagen
https://www.scienceimage.csiro.au/tag/laboratories/i/293/cells-interacting-with-collagen/